Global Scans · Synthetic Biology & Biotechnology · Signal Scanner
Emerging Disruption: The Confluence of Synthetic Biology and AI in Precision Medicine
The integration of synthetic biology and artificial intelligence (AI) is poised to transform precision medicine, presenting a weak signal that could evolve into a disruptive trend impacting healthcare, agriculture, conservation, and beyond. This convergence may redefine how therapies, including gene editing and cell therapies, are developed and scaled—from rare diseases to widespread conditions—while also intersecting with regulatory, ethical, and geopolitical concerns. As synthetic biology advances rapidly with new AI-driven capabilities, it invites scrutiny of biases and assumptions about the pace and scope of future innovation across multiple industries.
What's Changing?
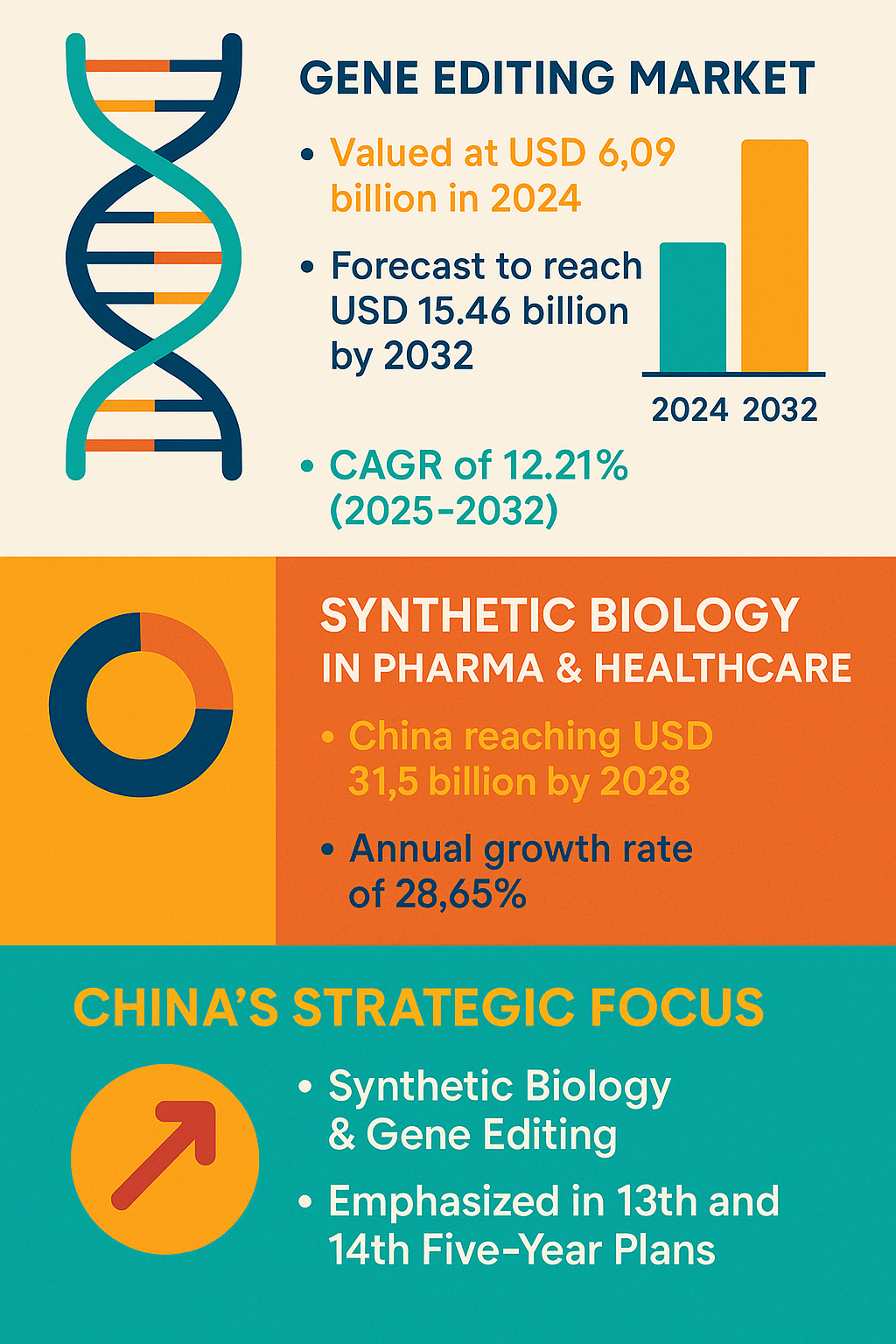
Synthetic biology—the design and construction of new biological parts and systems—has gained global strategic emphasis, notably in China’s 13th and 14th Five-Year Plans, positioning it as a priority for national innovation ecosystems (PMC NCBI). This focus signals a broader geopolitical dimension to biotechnological development that could drive accelerated research and commercial applications.
Recent advances in gene editing tools such as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) may soon move beyond treating rare diseases to targeting common chronic conditions, prognostically by 2028 (Ian Khan). This shift will likely expand the demand for scalable and adaptive therapeutic platforms, requiring innovations in precision medicine to tailor interventions closely to the patient's biology.
Meanwhile, AI technologies, especially deep learning algorithms, are rapidly advancing applications such as microbiome prediction and nutrient absorption modeling, valued at over USD 750 million and projected to grow steadily by 2032 (CNHI News). The power of AI to analyze complex biological data is enhancing the precision and speed of synthetic biology research. For instance, AI can optimize gene editing outcomes, design synthetic genomes, and predict unintended side effects faster than traditional methods.
Cellular agriculture, involving the lab-grown production of meat and other biological materials, is another critical facet of synthetic biology with substantial investment momentum. Venture capital is expected to surpass USD 50 billion in the next five years, underpinning efforts to scale lab-grown products to commercial viability (Ian Khan). AI-driven synthetic biology techniques could streamline production processes and improve product consistency, potentially disrupting traditional agriculture and food supply chains.
At the same time, notable regulatory and ethical signals are emerging. Global conservation groups have proposed moratoriums on synthetic biology applications such as genetic engineering of wildlife, which while lacking legal enforcement, could carry significant reputational and operational consequences for biotech firms (New Scientist). This introduces questions about the societal readiness and governance frameworks surrounding synthetic biology’s deployment.
Why is this Important?
The intersection of synthetic biology and AI for precision medicine and cellular agriculture may fundamentally alter multiple industries. For healthcare providers and pharmaceutical companies, this trend could mean faster development times, reduced costs, and highly personalized treatment options for patients with chronic and complex illnesses. Specifically, CRISPR-based therapies, aided by AI, might offer viable cures for conditions like HIV, shifting the healthcare landscape from management to potential eradication (Chemistry World).
In agriculture and food production, the rapid scaling of cellular agriculture enabled by synthetic biology and AI-powered optimization might disrupt traditional farming, livestock industries, and associated supply chains. This could reduce environmental footprints and address food security issues but also risks economic displacement and geopolitical shifts linked to agricultural export dependencies.
Policy and regulatory frameworks are likely to lag behind these rapid technological advances, creating uncertainty and risk. Ethically charged moratoriums or bans, even if non-binding, may influence public opinion and corporate strategies globally. Firms and governments need to navigate this evolving landscape carefully to balance innovation with societal expectations and environmental stewardship.
Implications
Businesses, researchers, and policymakers should monitor this weak signal closely, as it carries the potential to precipitate systemic changes that extend beyond healthcare. The following implications are particularly salient:
- Cross-sector Collaboration: Effective integration of AI with synthetic biology requires partnerships across biotechnology, data science, regulatory agencies, and ethical governance bodies to develop balanced innovation ecosystems.
- Investment and Infrastructure: Expanding capabilities in synthetic biology will demand substantial capital expenditures and infrastructure upgrades, including biofoundries and high-throughput computational platforms to handle complex biological data at scale.
- Regulatory Strategy: Organizations should proactively engage with global regulatory bodies and contribute to frameworks that address biosafety, bioethics, and intellectual property, reducing future compliance uncertainty.
- Workforce Evolution: Emerging roles combining biology, engineering, and data science will need to be cultivated, emphasizing interdisciplinary skills and ethical literacy to steer development responsibly.
- Scenario Planning and Risk Assessment: Scenario planners should incorporate synthetic biology and AI convergence as a disruptive force capable of triggering second-order effects across supply chains, labor markets, and geopolitical alignments.
Preparation now may enable stakeholders to capitalize on emerging opportunities while mitigating risks associated with unforeseen social, environmental, or market backlash.
Questions
- How can organizations integrate AI and synthetic biology to accelerate innovation while maintaining ethical standards and public trust?
- What governance structures can be designed to address potential conflicts between rapid biotech advancements and conservation or environmental protections?
- What are the risks of geopolitical fragmentation in biotechnology development, and how might international cooperation be fostered despite strategic competition?
- How will traditional industries, such as agriculture and pharmaceuticals, need to adapt their value chains in response to synthetic biology-driven disruption?
- What workforce skills and educational models should be prioritized to build expertise at the intersection of biology, AI, and ethics?
Keywords
synthetic biology; artificial intelligence; precision medicine; gene editing; cellular agriculture; CRISPR; healthcare innovation; biosafety; biotech governance.
Bibliography
- An IUCN moratorium on synthetic biology would have no legal force, but it could still have far-reaching effects. New Scientist. https://www.newscientist.com/article/2498841-would-a-ban-on-genetic-engineering-of-wildlife-hamper-conservation/
- China has placed significant emphasis on synthetic biology and gene editing technologies, making them strategic priorities in the 13th and 14th Five-Year Plans. PMC NCBI. https://pmc.ncbi.nlm.nih.gov/articles/PMC12479303/
- Crispr-based therapies will be an integral component for any cure for HIV in the future. Chemistry World. https://www.chemistryworld.com/news/could-crispr-cure-hiv/4022429.article
- By adopting AI, CRISPR, precision medicine, and mRNA platforms, the best biotech companies are driving innovation that will define the next era of global healthcare. Biomorph Life Sciences. https://biomorphlifesciences.com/top-biotech-companies-to-watch-in-2025/
- Venture capital investment in cellular agriculture will exceed $50 billion over the next five years, driving accelerated innovation and scaling. Ian Khan. https://www.iankhan.com/lab-grown-meat-in-2035-my-predictions-as-a-technology-futurist-7/
- Deep Learning, the fastest-growing subsegment, is projected to surpass USD 750 million by 2032 due to adoption in microbiome prediction and nutrient absorption modeling. CNHINEWS. https://www.cnhinews.com/news/article_a7005ebe-bdad-5fd1-82c6-493564a82740.html
- By 2028, gene editing and cell therapies will move from rare diseases to common conditions. Ian Khan. https://www.iankhan.com/the-future-of-medicine-7-transformative-trends-that-will-redefine-healthcare-by-2035-3/